Translate this page into:
Is Serum PSA a Reliable Indicator to Omit Skeletal Scintigraphy Among Newly Diagnosed Prostate Cancer Patients?

*Corresponding author: Manishi L. Narayan,Head, Department of Nuclear Medicine, All India Institute of Medical Sciences, Rishikesh, India manishi.ln@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh P, Singhal T, Negi M, Narayan ML. Is serum PSA a reliable indicator to omit skeletal scintigraphy among newly diagnosed prostate cancer patients? Indian Cancer Awareness J 2022;1:53-8.
Abstract
Objectives:
Skeletal scintigraphy is most sensitive modality for detection of bone metastases in prostate cancer (PCa). Bone scintigraphy (BS) is currently not recommended for staging of PCa patients with serum prostate specific antigen (S.PSA) <10 ng/ml or in low-risk group (NCCN 2021, EAU-EANM-2020). This study aims to establish cutoff of S.PSA levels to predict metastatic bone disease in newly diagnosed treatment naive patients with carcinoma Prostate, in Uttarakhand region, India.
Materials and Methods:
We retrospectively reviewed 105 treatment naïve PCa patients referred to Nuclear Medicine Department, All India Institute of Medical Sciences, Rishikesh, for BS. We assessed association between S.PSA levels (performed within 6 weeks of imaging), Gleason Score (GS)/International Society of Urological Pathology (ISUP) grading and metastatic disease diagnosed on BS.
Results:
A total of 105 patients were included in this study with an average age of 69 ± 9.4 years (42–87 years). Out of 105 patients, 62 (59%) were positive and 43 (41%) patients were negative on BS for skeletal metastasis. According to S.PSA levels, patients were divided into five subgroups. On subgroup analysis, most of the patients with S.PSA of >100 were positive for metastasis on BS (83.7%) but a significant number of patients with S.PSA<10 were also positive for skeletal metastasis (46%–7/15) on BS.
Conclusion:
In current patient population, a high incidence of bone metastasis is noted even at low S.PSA levels and in low-risk groups. Hence, BS can be considered in carcinoma prostate patients even with PSA levels <10 ng/ml. Although, other parameters such as GS/ISUP grading, pathological grade and clinical stage should also be considered and individualised risk adapted strategy to be followed for initial staging.
Keywords
Bone scan
Carcinoma prostate
Prostate specific antigen
Gleason score
INTRODUCTION
In the past few decades, there has been a rise in the incidence of cancer worldwide, accounting for 208.3 million DALY in 2015. It can mainly be attributed to population aging, population growth and increasing incidence of risk factors. According to the WHO, prostate cancer (PCa) is the second most common cancer in men and fourth most common cancer overall.[1,2]
Earlier, the prevalence of PCa in India was thought to be low, but due to changing lifestyles, migration of population to urban areas, increased awareness and easy access to medical facilities; there has been an increase in the cases diagnosed.[3] The reported incidence of PCa in Indian population is 2.6 with a mortality rate of 2.0.[4]
PCa is the fifth leading cause of cancer death in men worldwide.[1] Mortality is high in patients with advanced stages of cancer. In advanced cases, incidence of skeletal metastasis is 65–75%. According to SEER database analysis of 3857 men with metastatic PCa, patients with bone metastasis have 1.5 times higher probability of death as compared to men with lymph node involvement only.[5] Up to 14% of PCa patients have bone metastasis at the time of presentation.[6]
Once diagnosis of metastatic PCa is made, the main focus of management changes from treatment of primary to the treatment of metastasis and prevention of skeletal-related events (SREs). Early detection of bone metastasis becomes crucial in patient management,[7] as quality of life and overall survival of the patients can be improved with the use of newer agents, such as the receptor activator of nuclear factor kappa B ligand inhibitor, Denosumab and bisphosphonates as they prevent SREs, especially when initiated early.
At present, available investigations to detect bone metastasis include computed tomography (CT), magnetic resonance imaging, bone scintigraphy (BS) and positron emission tomography (PET)/CT. 99mTc-MDP BS is most widely available option for initial screening of skeletal metastases in PCa. Most commonly used tracers for BS include 99m TcMDP (methylene diphosphonate) [Figure 1] and 99 m TcHDP (hydroxymethylene diphosphonate).[8] 99mTc-MDP is administered intravenously, which gets rapidly chemisorbed onto the hydroxyapatite crystals of the osseous matrix. About 50% of administered radiotracer remains bound to the osseous matrix and rest is cleared mainly by the kidneys. MDP uptake is usually seen at the sites of osteoblastic activity and it is greater at the sites of active osteogenesis, than at the normal mature bone.[9,10]
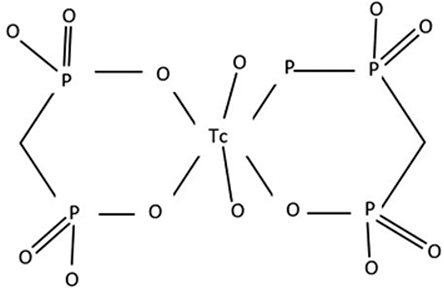
- Chemical structure of 99m Tc MDP.
Bone scan can detect altered metabolic activity much earlier than CT. Other advantages of BS over radiological modalities include its ability to screen the entire skeleton at low cost. Furthermore, it has higher sensitivity than skeletal radiography and serum alkaline phosphatase, for the detection of skeletal metastasis.[11]
At present, management of the patients with PCa is based on changes in serum prostate specific antigen (S.PSA) levels and presence of visceral or distant metastasis. BS helps in predicting the clinical stage of disease and treatment planning.
The incidence of bone metastasis is ~2% in patients with PSA <10 and 16% in those with PSA >20. It has been reported that the yield of BS is low in asymptomatic patients with PSA <10 and Gleason score (GS) <7; therefore, routine BS is not recommended in this subgroup of patients for initial staging. Bone metastasis can also be correlated with GS. Only 5% of patients with GS <6, whereas 30% with GS of >7 have been reported to have bone metastasis.[12] Bone metastasis is also more common in patients with high International Society of Urological Pathology (ISUP) grades than in patients with lowISUP grades.
According to current NCCN guidelines, BS is not recommended in patients with PSA <10. However, importance of early identification of bone metastasis has been supported by the RADAR group and European guidelines.[7,11,13,14] In our study, we have retrospectively correlated the presence or absence of bony metastasis on BS in patients with PCa with age, PSA levels and GS in population of Uttarakhand.
MATERIALS AND METHODS
We retrospectively reviewed treatment naïve PCa patients referred to the Department of Nuclear Medicine, A.I.I.M.S, Rishikesh, India, for BS. Patients with histologically proven PCa and S.PSA levels done within 6 weeks of BS were included in the study. The patients who had received any type of treatment affecting serum PSA levels such as hormonal therapy, chemotherapy, radiotherapy or surgical procedures such as orchidectomy and radical prostatectomy were excluded from the study.
We have assessed association between S.PSA levels (performed within 6 weeks of imaging), GS and metastatic disease on BS.
Data analysis
Statistical analysis was done using the Chi-square test and logistic regression analysis was used to compare the independent variable (PSA) and BS findings, by a statistical software (Statistical Package for the Social Sciences, version 26) and P < 0.05 is considered statistically significant.
RESULTS
A total of 105 patients referred for 99mTc MDP BS at A.I.I.M.S., Rishikesh, diagnosed with PCa and fulfilling our inclusion criteria were included in the study. The mean age of the study population was 69 ± 9.4 years (42–87 years). The GS ranged from 4 to 10 with a mean value of 8.0. Out of 105 patients, 62 (59%) were positive and 43 (41%) patients were negative on BS for skeletal metastases.
In both the groups, no significant difference was seen with age (P-value: 0.56) [Table 1]. However, PSA levels correlated significantly with the presence of bone metastasis (P-value: 0.003). Association with S.PSA, GS as well as ISUP grade with skeletal metastasis was evaluated. According to PSA levels, patients were divided into four groups: (I) Serum PSA <10 (n = 15), (II) Serum PSA >10 but <20 (n = 17), (III) Serum PSA 20–100 (n = 30) and (IV) Serum PSA value >100 (n = 43). Skeletal metastasis was present in 46.6%, 23.5%, 50% and 83.7% of patients in subgroups I, II, III and IV, respectively [Table 2].
| Age | 40–50 | 50–60 | 60–70 | 70–80 | >80 |
|---|---|---|---|---|---|
| No. of Patients | 1 | 13 | 38 | 34 | 19 |
| PSA value (ng/ml) | <10 (n=15) | 10–20 (n=17) | 20–100 (n=30) | >100 (n=43) |
|---|---|---|---|---|
| Patients with skeletal metastasis on BS (%) | 7 (46.6) | 4 (23.5) | 15 (50) | 36 (83.7) |
S. PSA: Serum prostate specific antigen, BS: Bone scintigraphy.
Group analysis revealed that patients with high-PSA levels had more chances of being positive for metastases on BS. On logistic regression analysis, although higher risk of bone metastasis (OR = 1.013, 95% CI = 1.004–1.022) is seen in patients with higher PSA levels, a significant percentage (46%) of patients with S.PSA <10 also showed skeletal metastases on BS. Logistic regression analysis did not yield significant results on, association of GS (P-value: 0.613) or patients age (P-value: 0.803) with bone metastasis [Table 3].
| Variables | P-value |
|---|---|
| PSA | 0.003 |
| HPE | 0.613 |
| Age | 0.803 |
| ISUP grade | 0.311 |
PSA: Prostate specific antigen, ISUP: International Society of Urological Pathology
Patients were also classified into three sub-groups, based on the GS. Patients with GS of >7 had higher risk of metastasis (64.2%) than those with GS of 7 (58.3%) or <7 (35.7%). However, this relationship was not statistically significant in our study population (P-value: 0.34) [Table 4 and Figure 2].
| Gleason’s score | Metastasis present (%) |
Metastasis absent (%) |
|---|---|---|
| <6 (n=14) | 5 (35.7) | 9 (64.3) |
| 7 (n=24) | 14 (58.3) | 10 (41.7) |
| >8 (n=67) | 43 (64.2) | 24 (35.8) |
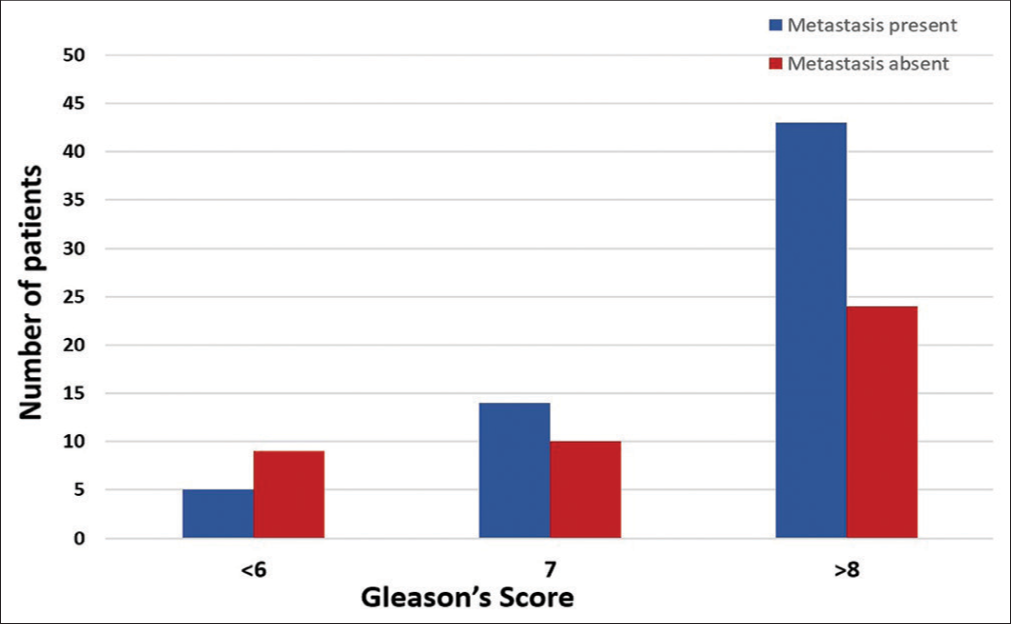
- Number of patients with bone metastasis with respect to Gleason’s score.
Based on histopathology, patients were assigned different grades as per ISUP grading of PCa. Incidence of bone metastasis on BS showed positive correlation with ISUP grade, though it was not statistically significant in our study (P-value: 0.311) [Table 5].
| Risk group | ISUP grade group | Gleason score | No. of patients | Metastasis present (%) |
|---|---|---|---|---|
| Low | Grade Group 1 | <6 | 14 | 5 (35.7) |
| Intermediate Favourable |
Grade Group 2 | 7 (3+4) | 10 | 5 (50) |
| Intermediate Unfavourable |
Grade Group 3 | 7 (4+3) | 14 | 9 (64.2) |
| High | Grade Group 4 | 8 | 20 | 14 (70) |
| High | Grade Group 5 | 9–10 | 47 | 29 (61.7) |
ISUP: International Society of Urological Pathology
DISCUSSION
BS is considered as the investigation of choice for screening, due to its higher sensitivity compared to radiological investigations and its ability to screen whole of skeleton in a single study. At present, BS is recommended only in patients with intermediate to high risk of metastasis. However, its use in low-risk groups is a matter of debate, particularly in Asian population. Many recent studies [Table 6] have reported higher rate of skeletal metastasis even at lower PSA levels in Asian population.[15-22] The incidence of metastatic PCa is about 1.7–11.9 but varies widely from region to region.[23]
| Study | Number of patients | PSA (ng/ml) |
Bone metastasis positive | |
|---|---|---|---|---|
| Number | % | |||
| Ito et al. (2000) | 303 | <10 | 13/36 | 36.1 |
| Yang et al. (2009) | 77 | <20 | 5/27 | 19.2 |
| Sanjaya et al. (2013) | 358 | <20 | 25/90 | 27.7 |
| <10 | 10/42 | 23.8 | ||
| Sharma et al. (2017) | 89 | <10 | 8/32 | 25 |
| 11–20 | 2/9 | 22.5 | ||
| Bhargava et al. (2018) | 85 | <20 | 11/31 | 35.48 |
| Singh et al. (2019) | 68 | <20 | 5/17 | 29.4 |
| Das et al. (2021) | 45 | <20 | 1/5 | 25 |
PSA: Prostate specific antigen
The most common site of distant metastasis is bone and is usually associated with poor prognosis. In the literature, the prognosis of PCa has been widely studied and prognosis remains grave across all the studies.[24-26] The survival rates for 1 and 5 years were approximately 47% and 3%, respectively, for patients with metastatic PCa at initial diagnosis, as reported by Nørgaard et al., compared to 5-year survival rate of 100% for localised disease.[27] The striking difference in overall survival makes early diagnosis of metastatic PCa, of utmost important.
The most common tumour marker used for screening of PCa and follow-up of patients with PCa is serum PSA levels. Oesterling et al.[28] were the first to address the possibility of serum PSA levels being able to predict BS results. They concluded that omitting BS for PSA <10 ng/ml, was a safe option.
There are many investigations available to detect metastasis in PCa including 18-F NaF PET/CT, 68-Ga PSMA PET/CT and 18-F-FDG PET/CT scan. These new targeted tracers can detect metastatic PCa with higher sensitivity and specificity than radiological investigations and conventional BS. However, due to their limited availability and higher cost, 99mTc-MDP bone scan is still considered as an investigation of choice for detection of skeletal metastasis.
According to American Urological Association (AUA) and the European Association of Urology (EAU), BS is recommended only in patients in intermediate to high-risk groups with PSA ≥10 ng/ml, even in patients with well-differentiated tumours; however, BS can safely be omitted in low-risk category with PSA <10 ng/ml.[29,30]
There are notable differences in the incidence rates and stage, at initial presentation among different geographical regions worldwide. Hence, guidelines based on data from Western nations cannot be indiscriminately applied to Asian countries.[31,32] At initial presentation, poorly differentiated carcinoma is more common in Asian population and about twice the number of patients present with GS >8 when compared to Western population.[15-17]
In India, the incidence of PCa has increased in the past decade considerably and there is constant debate whether BS should be used in all patients indiscriminately. Many studies have shown an increased risk of metastatic disease in Indian population even at the lower PSA levels [Figure 3].[15,17,21]

- 99mTc-MDP Bone scintigraphy done for initial staging in a recently diagnosed case of prostate cancer, showing multiple skeletal metastasis. This patient had S.PSA of <10 ng/ml and a Gleason’s score of 7, at the time of imaging.
Our study also showed similar trend with a total of about 59% (62/105) of patients had bone metastasis. On subgroup analysis, patients with PSA level >100 had significantly high risk of metastasis on BS (83.7%) but a considerable number of patients with S.PSA <10 were also positive for skeletal metastases (46%) on BS. Risk predictability correlated more with PSA levels than GS (P = 0.003 vs. 0.347).
The limitations of the present study include small sample size and selection bias, because data are collected from a tertiary care centre. Patients would have been referred here, at advanced stage of the disease and, therefore, had higher chances of having metastatic disease.
CONCLUSION
Considering our findings, we hereby conclude that, for primary staging in PCa, the recommendation for bone scan could possibly be modified, because a high incidence of metastasis is seen, even with lower PSA levels in our population. Serum PSA levels along with other risk factors like Gleason’s score, ISUP grade, age, presence of symptoms should also be considered prior to selecting patients for skeletal screening with bone scintigraphy. International (EAU/AUA), clinical management guidelines for initial staging for metastases should be considered with caution, particularly in Asian population to avoid under staging in PCa. Individualized risk adapted strategy to be considered for initial screening of skeletal metastasis in prostate cancer.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- PRESS RELEASE N° 263. 2018. Available from: https://www.gco.iarc.fr [Last accessed on 2021 Feb 24]
- [Google Scholar]
- Global Cancer Data: GLOBOCAN 2018. Geneva: Union for International Cancer Control; 208 Available from: https://www.uicc.org/news/global-cancer-data-globocan-2018 [Last accessed on 2021 Feb 24]
- [Google Scholar]
- Asian Pacific Journal of Cancer Prevention. Available from: https://www.journal.waocp.org/?sid=entrez:pubmed&id=pmid:24175761&key=2013.14.9.4973 [Last accessed on 2021 Aug 11]
- [Google Scholar]
- India Fact Sheet: Globocan 2020. Vol 361. Lyon, France: International Agency for Research on Cancer; 2020. p. :2.
- [Google Scholar]
- Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol. 2015;68:325-34.
- [CrossRef] [PubMed] [Google Scholar]
- Early identification and intervention matters: A comprehensive review of current evidence and recommendations for the monitoring of bone health in patients with cancer. Cancer Treat Rev. 2017;61:23-34.
- [CrossRef] [PubMed] [Google Scholar]
- 99mTc-Methyl diphosphonate In: Molecular Imaging and Contrast Agent Database (MICAD). Bethesda (MD): National Center for Biotechnology Information (US); 2004.
- [Google Scholar]
- Nuclear Medicine and Molecular Imaging: The Requisites. Available from: https://www.elsevier.com/books/nuclear-medicine-and-molecular-imaging-the-requisites/omalley/978-0-323-53037-8 [Last accessed on 2021 Jun 21]
- [Google Scholar]
- The Pathophysiologic Basis of Nuclear Medicine Vol 246. (2nd ed). Berlin, Germany: Springer Berlin Heidelberg; 2008. p. :365.
- [CrossRef] [Google Scholar]
- Bone Metastases and Mortality in Prostate Cancer. Can We Be Doing More? [Online]. Available from: https://www.urotoday.com/journal/everyday-urology-oncologyinsights/articles/92382-bone-metastases-and-mortality-in-prostate-cancer-can-we-be-doing-more-everyday-urology-full-text-article.html
- [Google Scholar]
- Appropriate use criteria for bone scintigraphy in prostate and breast cancer: Summary and excerpts. J Nucl Med. 2017;58:14N-7.
- [Google Scholar]
- A clinician's guide to next generation imaging in patients with advanced prostate cancer (RADAR III) J Urol. 2019;201:682-92.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.nccn.org/professionals/physician_gls/default.aspx#site [Last accessed on 2019 Dec 8]
- Role of serum prostate-specific antigen as predictor for bone metastases in newly diagnosed prostate cancer. J Cancer Res Ther. 2019;15(Supplement):S39-41.
- [CrossRef] [PubMed] [Google Scholar]
- Asian race and impact on outcomes after radical radiotherapy for localized prostate cancer. J Urol. 2003;170:901-4.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between serum prostate specific antigen levels with incidence of bone metastases in newly diagnosed prostate cancer patients in Indian population. JMSR. 2017;5:18297-303.
- [CrossRef] [Google Scholar]
- Correlation of prostate-specific antigen before prostate cancer detection and clinicopathologic features: Evaluation of mass screening populations. Urology. 2000;55:705-9.
- [CrossRef] [Google Scholar]
- The diagnostic correlations of bone scintigraphy, pathological grade and PSA for metastatic prostate cancers. J Clin Oncol. 2009;8:702-4.
- [CrossRef] [Google Scholar]
- Correlation between low Gleason score and prostate specific antigen levels with incidence of bone metastases in prostate cancer patients: When to omit bone scans? Asian Pac J Cancer Prev. 2013;14:4973-6.
- [CrossRef] [PubMed] [Google Scholar]
- A prospective study on incidence of bone metastases at various prostate specific antigen levels in carcinoma prostate in South Indian population. Int J Contemp Med Res. 2018;5:1-4.
- [Google Scholar]
- Correlation of prostate specific antigen, gleason score and bone metastases in bone scan in prostate cancer patient. SAS J Med. 2021;7:85-9.
- [CrossRef] [Google Scholar]
- Sites of synchronous distant metastases and prognosis in prostate cancer patients with bone metastases at initial diagnosis: A population-based study of 16,643 patients. Clin Transl Med. 2019;8:30.
- [CrossRef] [PubMed] [Google Scholar]
- Who dies from prostate cancer? Prostate Cancer Prostatic Dis. 2014;17:348-52.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment patterns of prostate cancer with bone metastasis in Beijing: A real-world study using data from an administrative claims database. Pharmacoepidemiol Drug Saf. 2019;28:1501-9.
- [CrossRef] [PubMed] [Google Scholar]
- Survival analysis and development of a prognostic nomogram for bone-metastatic prostate cancer patients: A single-center experience in Indonesia. Int J Urol. 2019;26:83-9.
- [CrossRef] [PubMed] [Google Scholar]
- Skeletal related events, bone metastasis and survival of prostate cancer: A population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162-7.
- [CrossRef] [PubMed] [Google Scholar]
- Serum prostate-specific antigen in a community-based population of healthy men: Establishment of age-specific reference ranges. JAMA. 1993;270:860-4.
- [CrossRef] [PubMed] [Google Scholar]
- EAU Guidelines: Prostate Cancer-Uroweb. Available from: https://www.uroweb.org/guideline/prostate-cancer [Last accessed on 2021 Aug 23]
- [Google Scholar]
- Prostate Cancer: Early Detection Guideline-American Urological Association. Available from: https://www.auanet.org/guidelines/guidelines/prostate-cancer-early-detection-guideline [Last accessed on 2021 Aug 23]
- [Google Scholar]
- Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part II: Individual countries. BJU Int. 2002;90:174-84.
- [CrossRef] [PubMed] [Google Scholar]
- Metastasis on bone scan with low prostate specific antigen (≤20 ng/ml) and Gleason's score (<8) in newly diagnosed Pakistani males with prostate cancer: Should we follow Western guidelines? Asian Pac J Cancer Prev. 2011;12:1529-32.
- [Google Scholar]







