Translate this page into:
Cytotoxicity in Tobacco Chewers: Insights from Buccal Cytome Analysis in Botad, Gujarat: A Cross-Sectional Study

*Corresponding author: Sanman Samova, Department of Science and Technology (DST), Gujarat Biotechnology Research Centre (GBRC), Gandhinagar, Gujarat, India. samova.sanman@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Doctor H, Yadav R, Samova S. Cytotoxicity in Tobacco Chewers: Insights from Buccal Cytome Analysis in Botad, Gujarat: A Cross-Sectional Study. Indian Cancer Awareness J. 2024;3:38-46. doi: 10.25259/ICAJ_11_2024
Abstract
Objectives:
Oral submucous fibrosis (OSMF) is a significant public health concern linked to tobacco consumption, particularly affecting rural populations in Gujarat. This condition, which can lead to serious health complications, underscores the urgent need for awareness and intervention strategies in these areas. The primary aim of this study was to investigate the prevalence of OSMF among tobacco chewers in rural Botad, focusing on identifying cellular abnormalities through buccal cytome analysis.
Materials and Methods:
A proforma-based approach was employed to collect data from OSMF patients. The study included buccal cytome analysis to assess cellular abnormalities among tobacco chewers, with a particular focus on those consuming mava and pan masala. Additionally, physical observations were made to correlate perinatal health with pre-cancerous lesions.
Results:
The findings revealed a predominance of OSMF in male patients from rural areas, highlighting a significant lack of awareness regarding the risks associated with tobacco use. Buccal cytome analysis indicated notable cellular abnormalities, especially among mava and pan masala consumers, suggesting heightened cytotoxicity. The study also found major abnormalities in tobacco chewers using lime.
Conclusion:
This study underscores the critical need for early detection, intervention, and public health initiatives to combat the prevalence and adverse effects of OSMF among tobacco chewers in rural Botad. Addressing this preventable condition is essential for improving community well-being and reducing the burden of tobacco-related health issues..
Keywords
Tobacco chewers
Buccal cytome analysis
Cytogenetic damage
Oral health
Oral health
Manifestations
INTRODUCTION
Tobacco consumption is a significant global health issue, with over 8 million people dying each year from tobacco-related diseases, including 1.3 million non-smokers exposed to second-hand smoke.[1,2] The majority of tobacco users live in low- and middle-income countries, where the burden of tobacco-related illness and death is heaviest.[3] Tobacco use is a leading preventable cause of death, killing nearly seven million people worldwide each year.[4] The tobacco epidemic is particularly critical in India, which is the second-largest consumer of tobacco globally, accounting for approximately one-sixth of the world’s tobacco-related deaths.[5]
Tobacco control measures are effective in reducing smoking prevalence, particularly when implemented simultaneously within a given country. These measures include smoking bans, health warnings, advertising bans, and tobacco taxes. For example, a tax increase that increases tobacco prices by 10% decreases tobacco consumption by about 4% in high-income countries and about 5% in low- and middle-income countries. Tobacco taxes are seen as the most cost-effective way of curbing tobacco use, particularly among youth and low-income populations.[6]
However, the implementation of tobacco control measures is uneven and limited, with higher-income countries having more anti-smoking laws in place than the low- and middle-income countries. Effective tobacco control requires sustained efforts from governments, including strengthening efforts on alternate cropping and alternate livelihoods to reduce tobacco use, raising public health awareness, and creating a mass movement against tobacco.[7,8]
Oral submucous fibrosis (OSMF) is a chronic, progressive condition characterized by fibrosis of the oral mucosa, causing limited mouth opening and difficulty in swallowing. This debilitating condition has been strongly linked to the chewing of areca nut and tobacco products, particularly in South Asia where the prevalence is high. Despite its potentially severe consequences, there is still limited understanding of the pathophysiology and risk factors associated with OSMF. Thus, this study aims to investigate the prevalence, clinical presentation, and potential risk factors of OSMF in frequent tobacco chewers. By examining a cohort of individuals who regularly consume tobacco, we hope to shed light on the factors contributing to the development of OSMF and inform public health interventions aimed at reducing its incidence.
Prevalence studies have unequivocally shown a concerning correlation between the consumption of tobacco in the form of chewing and the development of OSMF.[9-11] This debilitating condition predominantly affects individuals who regularly chew tobacco, betel nut, or areca nut preparations, with a considerably higher prevalence among the South Asian population due to cultural practices. The risk factors associated with the development of OSMF in tobacco chewers include the duration and frequency of chewing, coupled with the type of ingredients in the chewable formulations.[12,13]
OSMF is a potentially malignant disorder that predominantly affects individuals who consume areca nut and related products.[14] The etiology of OSMF is multifactorial, involving various components such as areca nut alkaloids, copper, iron, vitamin B complex deficiency, and genetic predisposition.[15,16] Areca nut, the primary causative agent, contains alkaloids like arecoline, which are cytotoxic and can induce fibrosis in the oral mucosa.[17,18] Chronic inflammation due to the abrasive nature of areca nut chewing leads to increased collagen deposition and decreased collagen degradation, resulting in fibrosis.[19,20] Moreover, copper and iron in areca nut further contribute to collagen cross-linking, exacerbating fibrosis.[21,22] Inadequate intake of vitamin B complex impairs collagen synthesis and repair processes, creating a conducive environment for fibrosis development. Genetic factors, including polymorphisms in genes related to collagen metabolism, may also play a role in susceptibility to OSMF progression. This intricate interplay of etiological factors underscores the complexity of OSMF pathogenesis, necessitating a comprehensive approach to its management and prevention.[23-26]
Clinical manifestations of OSMF include progressive difficulty in opening the mouth, a burning sensation in the oral cavity, altered taste sensation, and increased salivation.[9] Fibrosis of the oral mucosa leads to reduced mouth opening, known as trismus, which is a hallmark feature of OSMF. Other symptoms may include restricted tongue movement, pain while swallowing, and the presence of fibrous bands within the oral mucosa.[27-29] The diagnosis of OSMF is typically based on clinical examination and confirmed by histopathological analysis of biopsy samples from the affected area. Different staging systems have been proposed to classify the severity of OSMF, ranging from early to advanced stages based on the extent of fibrosis and functional impairment. Early detection and intervention are crucial to prevent disease progression and improve the quality of life for affected individuals.[30,31]
Management and treatment of OSMF involve a multidisciplinary approach aiming at halting disease progression and improving patients’ quality of life. The primary objective is to eliminate the causative agents, such as areca nut and tobacco, followed by symptomatic relief and rehabilitation. Traditional methods include discontinuation of habits, nutritional counseling, and physiotherapy to prevent trismus. Furthermore, pharmacological interventions like antioxidants, steroids, and hyaluronidase injections have been employed to reduce inflammation and fibrosis. Surgical procedures like fibrotomy or the release of fibrotic bands may be necessary in severe cases of limited mouth opening. However, early diagnosis and preventive measures play a crucial role in minimizing the severity of OSMF. Patient education and regular follow-ups are essential components of successful management strategies in combating this potentially debilitating condition.[29-31]
Objective
The objective of this study is to assess the prevalence and types of oral lesions among different groups of tobacco chewers (mava, pan masala, and sole tobacco users) and non-chewers in a rural area, evaluate overall oral health parameters and investigate the impact of various chewing habits. Additionally, the study aims to examine cytological changes in the buccal mucosa, analyze the association between tobacco chewing and pre-cancerous lesions, and provide demographic and behavioral insights into tobacco use.
MATERIALS AND METHODS
A total of 220 volunteers were recruited for this study. Volunteers were selected based on specific inclusion and exclusion criteria to ensure a representative sample of the population. Data collection was conducted through a combination of clinical examinations and questionnaire Customise proforma. Each participant underwent a comprehensive oral examination by a trained healthcare professional. In addition, participants completed a structured questionnaire to provide information on their medical history, lifestyle habits, and oral hygiene practices.
The examination process focused on identifying various types of oral lesions, including but not limited to leukoplakia, erythroplakia, oral submucous fibrosis and oral candidiasis. Lesions were classified based on their clinical appearance, location and size. In addition to assessing for oral lesions, participants were also examined for other oral health parameters, such as mouth opening status, presence of oral submucous fibrosis and signs of periodontal disease. These additional examinations provided a comprehensive evaluation of each participant’s oral health status.
Sample selection
We conducted a cross-sectional study focusing on buccal cytome analysis, enrolling apparently healthy individuals with varying chewing habits. Participants were divided into two main groups: (1) Tobacco chewers, based on their chewing practices, and (2) non-tobacco chewers. Among the chewers, subgroups were delineated according to specific chewing habits, including areca nut with tobacco and lime (mava), areca nut without tobacco (pan masala) and exclusive tobacco users. Non-tobacco chewers were considered as a control for the study.
Buccal cytome analysis
Buccal cells were obtained from both chewers and non-chewers, utilizing a modified methodology inspired by Belien et al., 1995.[32] One thousand differentiated cells were scored per subject for the various cell types outlined in the buccal cytome assay. These consisted of cells consisted micronucleus, binucleates, fragmented nuclei, pyknotic cells and the cell death parameters karyohectic, and karyolytic cells.
RESULTS
The present study aimed to investigate the comparative toxicity of mava and pan-masala in human subjects. This work follows a pro-forma-based approach. Based on a comprehensive literature review and preliminary investigation, the study was divided into two main parts:
Part 1: Assessment of oral sub-mucous fibrosis and precancerous lesions
The study highlights a significant correlation between poor oral hygiene and the occurrence of leukoplakia. Individuals with inadequate oral care practices were more likely to develop this pre-cancerous condition [Figure 1]. These findings emphasize the importance of maintaining proper oral hygiene to reduce the risk of leukoplakia.
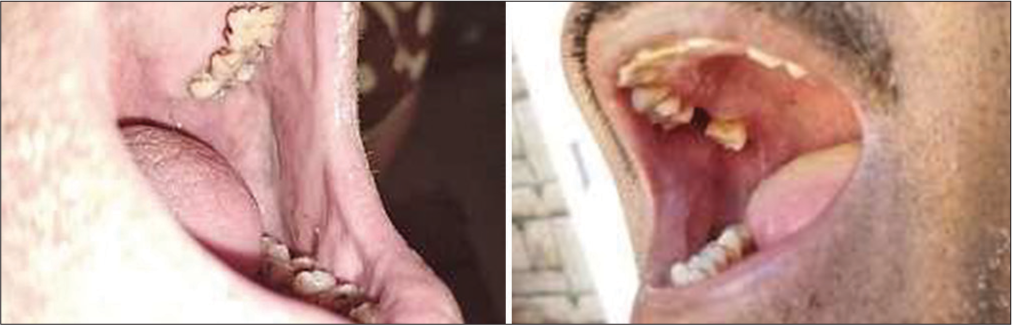
- Bad oral hygiene and leukoplakia.
The study highlights a significant correlation between difficulty in wide mouth opening and the presence of erythroplakia among tobacco chewers. Individuals with oral submucous fibrosis frequently exhibit restricted mouth opening, a condition exacerbated by the development of erythroplakia [Figure 2]. This finding underscores the compounded oral health challenges faced by tobacco users, emphasizing the need for targeted interventions and early diagnosis [Figures 1 and 2].
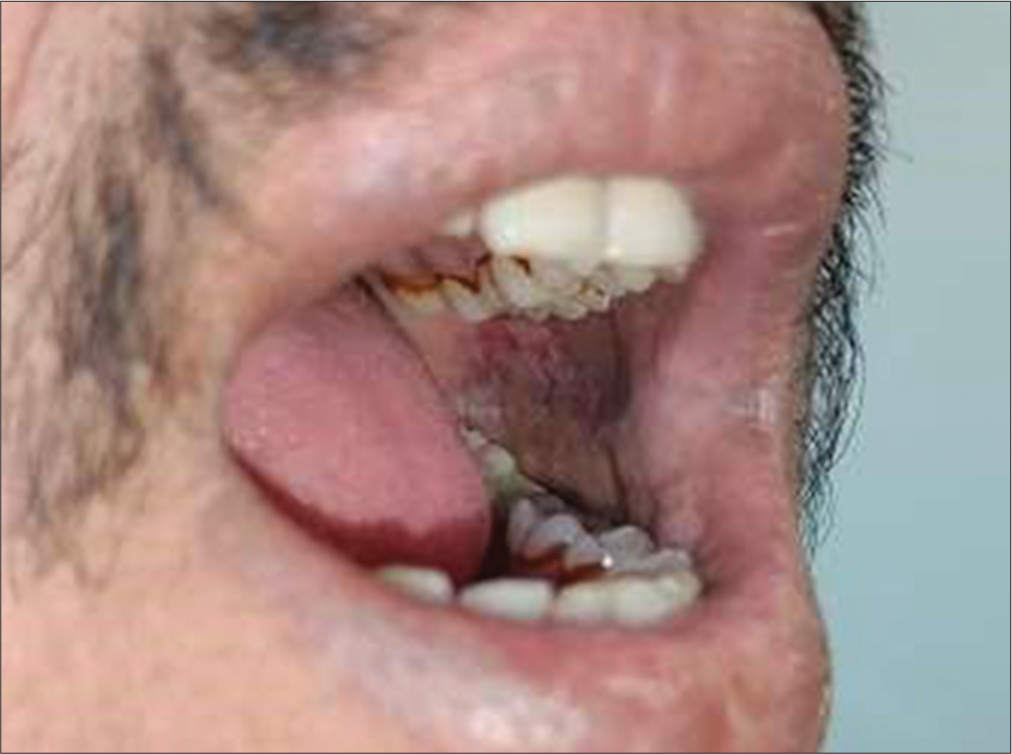
- Difficulty in wide mouth opening and erythroplakia.
Table 1 presents data on enrolled samples, categorised into control and tobacco-chewer groups, along with the presence of pre-cancerous lesions among tobacco chewers. Among the total 220 enrolled samples, the control group comprises 9.09% (20) of the samples, while the vast majority, constituting 90.91% (200), are tobacco chewers. Notably, among the tobacco chewers, 35% (70) exhibit pre-cancerous lesions. This breakdown underscores the significant prevalence of tobacco chewing within the sample population and highlights the concerning association between tobacco use and the development of pre-cancerous conditions.
| Total no samples | Control samples | Tobacco chewers | |
|---|---|---|---|
| 220 | 20 (9.09%) | 200 (90.91%) | |
| Tobacco chewers with pre-cancerous lesions | Tobacco chewers without pre-cancerous lesions | ||
| 70 35% | 130 (65%) | ||
Table 2 presents data on tobacco consumption duration categorised by years and gender. Among the total of 200 individuals, 8.5% (17) have consumed tobacco for less than or equal to one year, with males constituting the majority at 94.1% (16). For the range of 2–5 years, 43.5% (87) of individuals fall into this category, with males comprising 91.95% (80) and females 8.05% (7). In the 6–10 years category, 37.5% (75) of individuals have consumed tobacco, with males representing 97.33% (73) and females 2.67% (2). Those with more than 10 years of tobacco consumption constitute 10.5% (21) of the total, with males accounting for 90.48% (19) and females 9.52% (2). Overall, most individuals exhibit longer durations of tobacco consumption, with males dominating each category.
| Tobacco consumptions (years) | Male (%) | Female (%) | Total (%) |
|---|---|---|---|
| ≤ 1 year | 16 (94.1) | 1 (5.9) | 17 (8.5) |
| 2–5 | 80 (91.95) | 7 (8.05) | 87 (43.5) |
| 6–10 | 73 (97.33) | 3 (2.67) | 75 (37.5) |
| More then 10 | 19 (90.48) | 2 (9.52) | 21 (10.5) |
Table 3 illustrates data on tobacco chewers categorised by the type of tobacco product that they consume. Among the total 200 tobacco chewers, the majority, comprising 48.5% (97), consume areca nut with tobacco and lime, commonly known as ‘mava’. In addition, 37.5% (75) of tobacco chewers prefer areca nut without tobacco, referred to as ‘pan masala’. The remaining 14% (28) of tobacco chewers consume sole tobacco. This breakdown reveals the varied preferences among tobacco chewers regarding the type of tobacco product they choose, with a significant proportion favouring areca nut-based products such as mava or pan masala.
| Tobacco chewers | Areca nut With tobacco and lime (Mava) | Areca nut without tobacco (Pan masala) | Sole tobacco |
|---|---|---|---|
| 200 | 97 (48.5%) | 75 (37.5%) | 28 (14%) |
Assessing oral lesions and chewing habits: Implications for mucosal health and potential precancerous changes
A cross-sectional observational study was undertaken among ostensibly healthy subjects exhibiting various chewing habits. Notably, individuals categorised as frequent chewers displayed discernible clinical manifestations characterised by the presence of white lesions, identified as leukoplakia and red lesions, recognised as erythroplakia. These lesions serve as clinical indicators of potential mucosal abnormalities associated with chronic chewing habits, thereby warranting further investigation into their aetiology and potential progression toward malignancy.
In the study of 200 tobacco chewers, 35% (70) were identified to have pre-cancerous lesions. Among these individuals, 18% (36) experienced difficulty in wide mouth opening, while 6% (12) exhibited erythroplakia, characterised by red lesions. In addition, 7.5% (15) of the tobacco chewers displayed leukoplakia, which presents as white patches. These findings underscore the prevalence of pre-cancerous lesions among tobacco chewers, with a notable proportion experiencing clinical manifestations such as difficulty in mouth opening and various types of oral lesions.
Among individuals with a habitual chewing practice involving either areca nut or tobacco, a markedly reduced incidence of precancerous lesions was observed in comparison to those with alternative chewing habits. Furthermore, the diversity of lesion types exhibited notably lower variability among individuals consuming areca nut or tobacco as opposed to those engaging in the consumption of pan masala or mava.
Part 2: Evaluation of cytological changes in buccal mucosa cells among chewers
The evaluation of cytological changes in buccal mucosa cells among chewers reveals significant alterations. These include increased occurrences of binucleate cells, ruptured and fragmented nuclei, and pyknotic cells. Such findings underscore the adverse cellular effects associated with habitual chewing, highlighting the potential for serious oral health issues. Figure 3 illustrates various cellular conditions: (a) a normal cell; (b and c) binucleate cells; (d) a cell with a ruptured nucleus; (e) a cell with a fragmented nucleus; and (f) a pyknotic cell. These images highlight the spectrum of cytological changes, ranging from normal morphology to significant nuclear alterations. Such variations are critical for understanding the cellular impact of different pathological states.
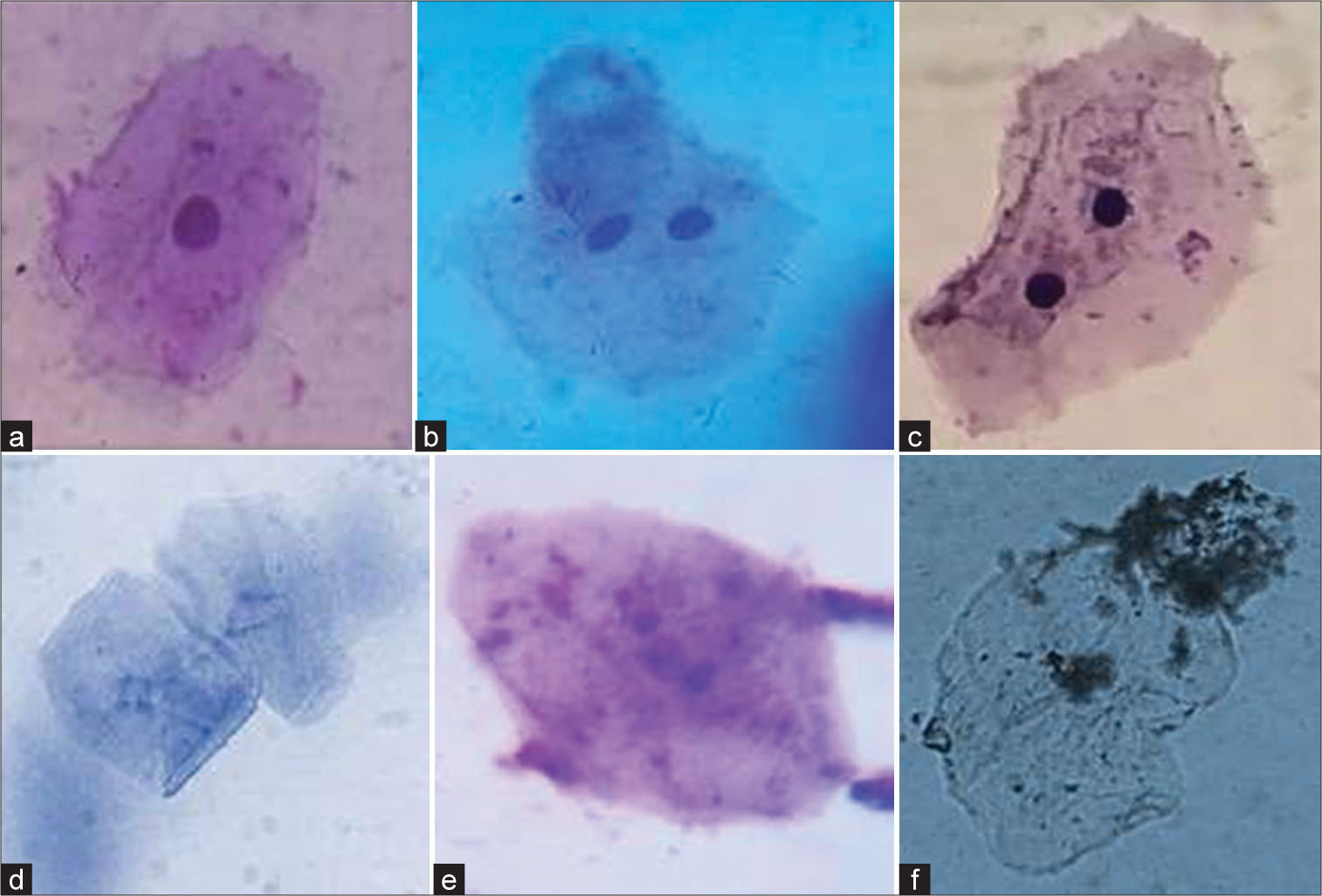
- (a) A normal cell; (b and c) binuates; (d) ruptured nucleus; (e) fragmented nucleus and (f) pycnotic cell.
The results revealed a higher frequency of binucleated and micronucleated cells compared to other anomalies. Pan masala consumers displayed the highest incidence of binucleated cells, while tobacco consumers exhibited the highest occurrence of micronuclei, followed closely by areca nut consumers.
Table 4 displays the average number of binucleates and micronuclei, abnormal cells indicative of potential cytogenetic damage, observed in various experimental groups compared to a control group. In comparison to the control, which exhibited minimal occurrences of both binucleates and micronuclei at 0.2, the experimental groups displayed substantially higher values. Mava chewers exhibited a staggering increase, with binucleated and micronuclei reaching averages of 14.400 ± 2.22 and 12.600 ± 1.78, respectively. Similarly, individuals consuming pan masala and solo tobacco also showed considerable elevations in both types of abnormal cells, with values ranging from 13.100 to 13.700 for binucleated and 10.900 ± 1.97–11.800 ± 1.75 for micronuclei. These findings suggest a pronounced cytogenetic impact associated with mava, pan masala and solo tobacco consumption, underscoring potential health risks associated with these habits.
| Binucleates | Micronucleus | Karyolitic cell | Picknotic cell | Ruptured nucleus | |
|---|---|---|---|---|---|
| Control | 0.200±0.42 | 0.200±0.42 | 0.100±0.32 | 0.100±0.32 | 0.200±0.42 |
| Mava | 14.400±2.22a | 12.600±1.78a | 5.300±1.49a | 10.100±1.45a | 7.600±2.01a |
| Pan masala | 13.100±2.28a | 10.900±1.97a | 4.600±0.97a | 7.800±1.81a | 6.100±2.42a |
| Solo tobacco | 13.700±2.71a | 11.800±1.75a | 4.300±1.23a | 9.200±1.23a | 6.300±2.50a |
Values are expressed as mean±standard error of the mean; The mean difference is significant at the 0.05 level. aAs compared to control.
Regarding cellular death parameters such as karyohexis, karyolysis and pykotic cells, a significant elevation was observed in groups consuming mava and pan masala. Mava consumption demonstrated a notably higher prevalence of karyohexis followed by tobacco consumption. Karyolysis was most pronounced in buccal cells of mava consumers, whereas cells with pykotic nuclei were more abundant in tobacco consumers, followed by mava consumers. In addition, mava consumers exhibited the highest occurrence of ruptured and fragmented nuclei.
While areca nut consumers displayed cellular abnormalities compared to normal individuals, the incidence was comparatively lower than that observed in other forms of chewing habits.
DISCUSSION
Oral submucous fibrosis (OSMF) prevails prominently in Gujarat, particularly among individuals engaged in areca nut and tobacco chewing practices.[10] OSMF disrupts oral homeostasis, leading to symptoms such as dysphagia, xerostomia, and restricted mouth opening. Diagnosis of OSMF often necessitates molecular pathology methods, which may involve invasive procedures to identify biomarkers.[31,33]
A demographic analysis of OSMF patients in Botad revealed a predominance of males, with the vast majority hailing from rural areas and belonging to the literate demographic. While patients exhibited diverse occupations, a significant portion were engaged in physical labor. The study identified a concerning lack of knowledge, attitude, and practice regarding areca nut, gutka, and tobacco consumption among the young population in Botad. Urgent interventions, including community awareness programs targeting school children and the general public, are imperative to address this issue effectively.[1,34,35]
Despite efforts by “nasha mukti kendras” in schools and colleges, a substantial proportion of the male population in Botad remains addicted to tobacco chewing. Both rural and urban residents exhibit conservatism and reluctance to participate in health-related studies due to low literacy levels. The availability of non-invasive techniques like VELscope for cancer detection offers a promising avenue, yet public awareness and fear of cancer hinder early diagnosis and timely intervention, leading to advanced stages that are often incurable.[36-38]
Subjects who initiated tobacco consumption at a young age, particularly those under 21 and 25 reported weakness and abnormal body mass index, indicative of adverse health effects associated with tobacco consumption. Notably, parental indifference towards tobacco addiction among their sons perpetuates a societal acceptance of addiction, necessitating heightened attention from both the community and healthcare authorities.[39-41]
The study indicates a high prevalence of tobacco chewing within the sample population. Of the 220 enrolled samples, 90.91% (200) are tobacco chewers, while only 9.09% (20) constitute the control group. This finding suggests that tobacco chewing is a significant concern within this specific population. Furthermore, the table reveals a concerning association between tobacco chewing and pre-cancerous lesions. Among the tobacco chewers, 35% (18) exhibit pre-cancerous lesions. This observation aligns with established scientific evidence linking tobacco use, including chewing, to an increased risk of developing certain types of cancer, especially oral cancer. The high prevalence of tobacco chewing and its association with pre-cancerous lesions within this population raises significant concerns and underscores the need for targeted interventions. Public health initiatives aimed at reducing tobacco use and promoting oral health should be implemented to mitigate the risks associated with tobacco chewing.[42-44] The data presented in Table 2 highlight the prevalence of tobacco consumption duration among a group of 200 individuals, categorized by year and gender. A significant proportion of individuals (43.5%) have consumed tobacco for a duration of 2 to 5 years. Longer durations of tobacco consumption (6 to 10 years and more than 10 years) are also common, accounting for 37.5% and 10.5% of the sample, respectively. The majority of individuals in all consumption duration categories are male, ranging from 91.95% for 2 to 5 years to 97.33% for 6 to 10 years.[36,45]
Males are significantly more likely to consume tobacco for longer durations compared to females. In every consumption duration category, the proportion of males is higher than that of females. For durations of 1 year, 6–10 years and more than 10 years, the percentage of males is over 90%. The high prevalence of tobacco consumption, particularly among males, suggests a need for targeted public health interventions. These interventions should focus on reducing initiation and prolonging abstinence from tobacco use.[46,47]
The study sample size (200 individuals) is relatively small, which may limit the generalizability of the findings. The research did not explore the specific types of tobacco products consumed or the reasons for differences in consumption duration by gender.
The study presents data on the distribution of tobacco chewers based on their preferred tobacco product. Among the 200 tobacco chewers surveyed, a substantial 48.5% (97 respondents) reported consuming ‘mava’, a mixture of areca nut, tobacco, and lime. This finding suggests that mava is the most prevalent form of tobacco chewing among the study population. Another notable aspect of the data is the relatively high prevalence of consuming areca nuts without tobacco. This practice, known as ‘panmasala’, accounted for 37.5% (75 respondents) of tobacco chewers. This indicates a preference for areca nuts as a standalone chewing substance, likely due to their stimulating and addictive properties.
In contrast, consuming sole tobacco was reported by a minority of tobacco chewers, comprising only 14% (28 respondents). This suggests that mixing tobacco with other substances, such as areca nut or lime, is more common than using tobacco alone.
The study reveals significant heterogeneity in tobacco chewing preferences among the study population. Arecanut-based products, particularly mava and pan masala, emerge as the most widely consumed forms of tobacco. Understanding these preferences is crucial for developing effective tobacco control strategies that cater to the specific needs and behaviours of different tobacco users.
Binucleates are cells with two nuclei, typically resulting from mitotic failure. Micronuclei are small, acentric nuclear fragments generated during cell division, often due to chromosomal breakage and rearrangements. The presence of these abnormal cells is considered a sensitive indicator of potential cytogenetic damage, suggesting genotoxicity and increased cancer risk. [48,49] The elevated levels of binucleates and micronuclei observed in mava, pan masala and solo tobacco consumers strongly suggest a harmful cytogenetic impact associated with these habits. Several studies have identified various toxic and mutagenic compounds in these substances, including nicotine, tar and polycyclic aromatic hydrocarbons. These compounds can interact with deoxyribonucleic acid (DNA), leading to structural damage, chromosomal aberrations and ultimately increased cancer susceptibility.[37]
Table 4 showcases a comparative analysis of the average number of binucleates and micronuclei in experimental groups versus a control group. The control group exhibited minimal occurrences of both binucleates (0.2) and micronuclei (0.2). However, the experimental groups displayed significantly higher values across the board. Mava chewers exhibited the most pronounced increase, with binucleated and micronuclei reaching averages of 14.400 ± 2.22 and 12.600 ± 1.78, respectively. Pan masala and solo tobacco consumers also showed considerable elevations in both types of abnormal cells, with values ranging from 13.100 to 13.700 for binucleated and 10.900 ± 1.97–11.800 ± 1.75 for micronuclei. Furthermore, the presence of betel quid in mava and pan masala introduces additional cytogenetic risks. Betel quid contains arecoline, a known carcinogen that has been linked to DNA damage and genomic instability.[50,51]
The findings presented in Table 4 provide compelling evidence for the cytogenetic effects of mava, pan masala and solo tobacco consumption. The observed increase in binucleates and micronuclei suggests a profound impact on chromosomal health, potentially leading to increased risks of cancer and other severe health conditions. These findings underscore the critical need for tobacco cessation interventions and public awareness campaigns to discourage these harmful habits and promote healthier lifestyles.[52,53]
CONCLUSION
The findings of our study on oral submucous fibrosis in frequent tobacco chewers highlight the pressing need for heightened awareness and interventions to address this preventable yet debilitating condition. Our research underscores the detrimental effects of long-term tobacco chewing on oral health, particularly in individuals who engage in this harmful behavior regularly. The pathophysiology of oral submucous fibrosis is complex and involves various biochemical and molecular mechanisms that contribute to the progressive fibrosis of the oral mucosa. Furthermore, our study emphasizes the importance of early detection and prompt intervention in managing oral submucous fibrosis to prevent its irreversible complications. Moving forward, public health initiatives aimed at tobacco cessation and oral health education are crucial in reducing the prevalence of this condition and improving the overall well-being of individuals at risk.
Acknowledgment
We deeply appreciate the Primary Health Center Salangpur and Dr. R.B. Sangani of Jivandhara Hospital for their invaluable assistance and expertise in patient counseling and sample collection. Their dedication and collaborative spirit have greatly improved our quality of work. We want to extend our sincere gratitude to Dr. R.J. Verma for his invaluable guidance throughout this study. His expertise and support have been helpful in the successful completion of our work
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- WHO Report on the Global Tobacco Epidemic, 2023: Protect People from Tobacco Smoke Geneva: WHO; 2023.
- [Google Scholar]
- Global, regional, and national mortality of tuberculosis attributable to alcohol and tobacco from 1990 to 2019: A modelling study based on the Global Burden of Disease study 2019. J Glob Health. 2024;14:4023.
- [CrossRef] [Google Scholar]
- Health Effects of Tobacco at the Global, Regional, and National Levels: Results From the 2019 Global Burden of Disease Study. Nicotine Tob Res. 2022;24:864-70.
- [CrossRef] [Google Scholar]
- World No Tobacco Day: Tobacco is a Threat to the One Health and Sustainability. Cien Saude Colet. 2020;25:4347-50.
- [CrossRef] [Google Scholar]
- The Progression of the Tobacco Epidemic in India on the National and Regional Level, 1998-2016. BMC Public Health. 2022;22:317.
- [CrossRef] [Google Scholar]
- Price, Income, and Affordability as the Determinants of Tobacco Consumption: A Practitioner's Guide to Tobacco Taxation. Nicotine Tob Res. 2021;23:40-7.
- [CrossRef] [Google Scholar]
- The Economics of Tobacco and Tobacco Control Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, and World Health Organization; 2016.
- [Google Scholar]
- An Overview of the Tobacco Problem in India. Indian J Med Paediatr Oncol. 2012;33:139-45.
- [CrossRef] [Google Scholar]
- Clinico-pathological Study of 170 Cases of Oral Sub-mucous Fibrosis. Int J Sci Stud. 2015;3:137-44.
- [Google Scholar]
- Oral Submucous Fibrosis: A Review on Etiopathogenesis, Diagnosis, and Therapy. Int J Mol Sci. 2019;20:2940.
- [CrossRef] [Google Scholar]
- Prevalence of Oral Submucous Fibrosis among Areca Nut Chewers: A Systematic Review and Meta-analysis. Oral Dis. 2023;29:1920-6.
- [CrossRef] [Google Scholar]
- Demographic and Clinical Profile of Oral Submucous Fibrosis: A Retrospective Study. J Pharm Res Int. 2021;33:308-17.
- [CrossRef] [Google Scholar]
- Oral Submucous Fibrosis: Etiological Mechanism, Malignant Transformation, Therapeutic Approaches and Targets. Int J Mol Sci. 2023;24:4992.
- [CrossRef] [Google Scholar]
- Areca Nut-induced Oral Fibrosis-Reassessing the Biology of Oral Submucous Fibrosis. J Oral Biosci. 2024;66:320-8.
- [CrossRef] [Google Scholar]
- Oral Submucous Fibrosis: A Review on Biomarkers, Pathogenic Mechanisms, and Treatments. Int J Mol Sci. 2020;21:7231.
- [CrossRef] [Google Scholar]
- Molecular Mechanism of Oral Submucous Fibrosis Induced by Arecoline: A Literature Review. J Clin Diagn Res. 2020;14:ZE01-5.
- [CrossRef] [Google Scholar]
- Carcinogenic Effects of Areca Nut and Its Metabolites: A Review of the Experimental Evidence. Clin Pract. 2023;13:326-46.
- [CrossRef] [Google Scholar]
- Antifibrotic Effect of Ocimum basilicum L. and Linalool on Arecoline-induced Fibrosis in Human Buccal Fibroblasts: An In vitro Study. Transl Res Oral Oncol. 2018;3:1-9.
- [CrossRef] [Google Scholar]
- The Role of Serum Copper and Iron in Oral Submucous Fibrosis. J Indian Acad Oral Med Radiol. 2004;16:30-2.
- [CrossRef] [Google Scholar]
- Oral Submucous Fibrosis: An Update on Current Theories of Pathogenesis. J Oral Pathol Med. 2017;46:406-12.
- [CrossRef] [Google Scholar]
- Estimation of Serum Iron and Serum Magnesium Levels in Oral Submucous Fibrosis Patients: A Comparative Study (Master's thesis In: Rajiv Gandhi University of Health Sciences (India). 2017.
- [Google Scholar]
- Evaluation of Hematological Profile in Oral Submucous Fibrosis: A Cross-sectional Study. J Oral Maxillofac Pathol. 2020;24:575.
- [CrossRef] [Google Scholar]
- The Role of Macronutrients and Micronutrients in Wound Healing: A Narrative Review. J Wound Care. 2022;31:S14-22.
- [CrossRef] [Google Scholar]
- Interaction of Collagen-related Genes and Susceptibility to Betel Quid-induced Oral Submucous Fibrosis. Cancer Epidemiol Biomarkers Prev. 2002;11:646-53.
- [Google Scholar]
- Genetic Susceptibility and Protein Expression of Extracellular Matrix Turnover-Related Genes in Oral Submucous Fibrosis. Int J Mol Sci. 2020;21:8104.
- [CrossRef] [Google Scholar]
- Various Oral Habits Associated with the Prevalence of Oral Submucous Fibrosis in a Western Uttar Pradesh Population: A Cross Sectional Demographic Study. EJPMR. 2022;9:250-4.
- [Google Scholar]
- Assessment of Correlation between Clinical Staging, Functional Staging, and Histopathological Grading of Oral Submucous Fibrosis. J Carcinog. 2021;20:16.
- [CrossRef] [Google Scholar]
- Oral Submucous Fibrosis: A Contemporary Narrative Review with a Proposed Inter-professional Approach for an Early Diagnosis and Clinical Management. J Otolaryngol Head Neck Surg. 2020;49:3.
- [CrossRef] [Google Scholar]
- Medicinal Management of Oral Submucous Fibrosis in the Past Decade-A Systematic Review. J Oral Biol Craniofac Res. 2020;10:552-68.
- [CrossRef] [Google Scholar]
- Exploring Possible Diagnostic Precancerous Biomarkers for Oral Submucous Fibrosis: A Narrative Review. Cancers. 2023;15:4812.
- [CrossRef] [Google Scholar]
- Standardization of Counting Micronuclei: Definition of a Protocol to Measure Genotoxic Damage in Human Exfoliated Cells. Carcinogenesis. 1995;16:2395-400.
- [CrossRef] [Google Scholar]
- Oral Submucous Fibrosis: A Global Challenge. Rising Incidence, Risk Factors, Management, and Research Priorities. Periodontology. 2000;80:200-12.
- [CrossRef] [Google Scholar]
- Interventions for Tobacco Prevention and Control in Humanitarian Settings: A Scoping Review. Nicotine Tob Res 2024:ntae135.
- [CrossRef] [Google Scholar]
- Awareness Regarding the Antitobacco Laws and Perceptions Regarding Interventions for Effective Tobacco Control among Young Adults Of Haryana, India. Indian J Public Health. 2023;67:92-8.
- [Google Scholar]
- Protecting Youth from Tobacco around the Globe: Evidence to Practice. Pediatrics. 2020;146:e20201585.
- [CrossRef] [Google Scholar]
- Evaluation of Micronuclei and Cytomorphometric Changes in Patients with Different Tobacco Related Habits Using Exfoliated Buccal Cells. Asian Pac J Cancer Prev. 2021;22:1851-5.
- [CrossRef] [Google Scholar]
- Betel Nuts, Health Policies, and Adolescent Health. Innov Digit Health Diagn Biomark. 2023;3:46-53.
- [CrossRef] [Google Scholar]
- Environmental Influences on Tobacco Use: Evidence from Societal and Community Influences on Tobacco Use and Dependence. Annu Rev Clin Psychol. 2009;5:433-58.
- [CrossRef] [Google Scholar]
- Predictors of Cigarette Smoking, Smokeless Tobacco Consumption, and Use of both forms in Adolescents in South Asia: A Secondary Analysis of the Global Youth Tobacco Surveys. Nicotine Tob Res. 2021;23:956-65.
- [CrossRef] [Google Scholar]
- Parental Support and Monitoring as Associated with Adolescent Alcohol and Tobacco Use by Gender and Age. BMC Public Health. 2021;21:2000.
- [CrossRef] [Google Scholar]
- Pancreatic cancer risk in relation to lifetime smoking patterns, tobacco type, and dose-response relationships. Cancer Epidemiol Biomark Prev. 2020;29:1009-18.
- [CrossRef] [Google Scholar]
- Effect of Areca Nut on Oral Health: A Review. J Res Dent Maxillofac Sci. 2020;5:1-6.
- [CrossRef] [Google Scholar]
- Effect of Frequency and Duration of Tobacco Use on Oral Mucosal Lesions-A Cross-Sectional Study among Tobacco Users in Hyderabad, India. Asian Pac J Cancer Prev. 2017;18:2233-8.
- [Google Scholar]
- Tobacco Cessation in Primary Care: Maximizing Intervention Strategies. Clin Med Res. 2003;1:201-16.
- [CrossRef] [Google Scholar]
- WHO Framework Convention on Tobacco Control: Development of An Evidence Based Global Public Health Treaty. BMJ. 2003;327:154-7.
- [CrossRef] [Google Scholar]
- State of Art of Micronuclei Assay in Exfoliative Cytology as a Clinical Biomarker of Genetic Damage in Oral Carcinogenesis: A Systematic Review and Meta-analysis. Mutat Res Rev Mutat Res. 2024;794:108508.
- [CrossRef] [Google Scholar]
- Cancers of the Oral Cavity In: Carcinogenicity. United States: CRC Press; 2021. p. :653-77. Available from: https://scholar.google.com/scholar?hl=en&as_sdt=0%2c5&q=saranath%2c+d.+%282021%29.+cancers+of+the+oral+cavity.+in+carcinogenicity+%28pp.+653-677%29.+crc+press.&btng [Last accessed on 2024 May 21]
- [CrossRef] [Google Scholar]
- Cytogenetic Alterations in Buccal Mucosa Cells of Chewers of Areca Nut and Tobacco. Arch Oral Biol. 2011;56:63-7.
- [CrossRef] [Google Scholar]
- Genetic Toxicology and Toxicokinetics of Arecoline and Related Areca Nut Compounds: An Updated Review. Arch Toxicol. 2021;95:375-93.
- [CrossRef] [Google Scholar]
- Micronuclei as Biomarkers of DNA Damage, Aneuploidy, Inducers of Chromosomal Hypermutation and as Sources of Pro-inflammatory DNA in Humans. Mutat Res Rev Mutat Res. 2020;786:108342.
- [CrossRef] [Google Scholar]
- Micronuclei and Genome Chaos: Changing the System Inheritance. Genes (Basel). 2019;10:366.
- [CrossRef] [Google Scholar]






